
Двојна природа микрочестица Физика и Оптика
This chemistry video tutorial explains how to calculate the de broglie wavelength of large objects and small particles such as electrons. It contains plenty.

PPT Chapter 5 PowerPoint Presentation, free download ID3201239
De Broglie Wavelength Matter waves are the central part of the theory of quantum mechanics. All matter can exhibit wave-like behaviour. The concept that matter behaves like a wave this concept was proposed by a French physicist named Louis de Broglie in the year 1924. It is also known as the de Broglie hypothesis.

De Broglie Wavelength Formula Equation, Concept, Solved Examples
De Broglie's hypothesis is an independent postulate about the structure of nature. In this respect, its status is the same as that of Newton's laws or the laws of thermodynamics. Nonetheless, we can construct a line of thought that is probably similar to de Broglie's, recognizing that these are heuristic arguments and not logical.

De Broglie Wavelength Formula Formula, Derivation, Applications
The de Broglie wavelength of the photon can be computed using the formula: λ = h p = 6.63×10−34 1.50×10−27 = 4.42 ×10−7 = 442 ×10−9 = 442 Nano meter. Therefore, the de Broglie wavelength of the photon will be 442 nm. This wavelength will be in the blue-violet part of the visible light spectrum.

Question Video Identifying the de Broglie Relationship Nagwa
de Broglie Wave Equation. Planck's investigation of the emission spectra of hot objects and the subsequent studies into the photoelectric effect had proven that light was capable of behaving both as a wave and as a particle. It seemed reasonable to wonder if electrons could also have a dual wave-particle nature. In 1924, French scientist Louis.

De Broglie's Formula YouTube
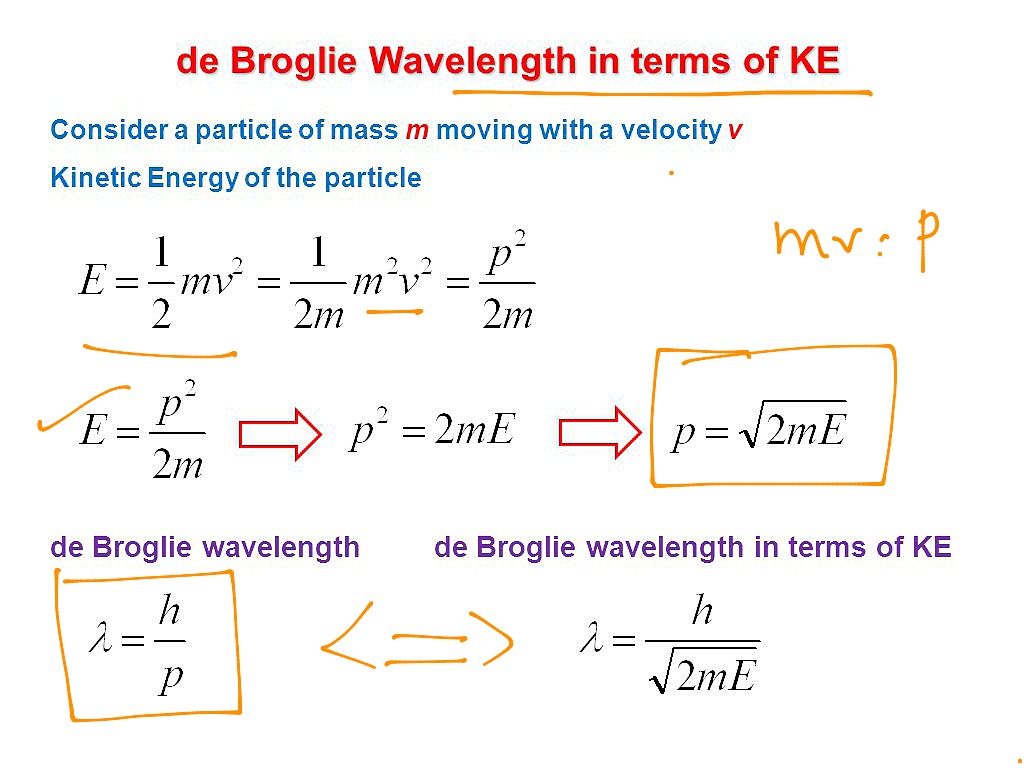
Solution: Reasoning: The de Broglie wavelength of an object is defined as λ = h/p. Details of the calculation: λ = h/p, E = p 2 / (2m), p = √ (2mE), λ = h/√ (2mE). The energy of the electron is 25000 eV * 1.6*10 -19 J/eV = 4*10 -15 J. λ = (6.626*10 -34 Js)/√ (2*9.1*10 -31 kg*4*10 -15 J) = 7.8*10 -12 m.
De Broglie wave equation Derivation by SK
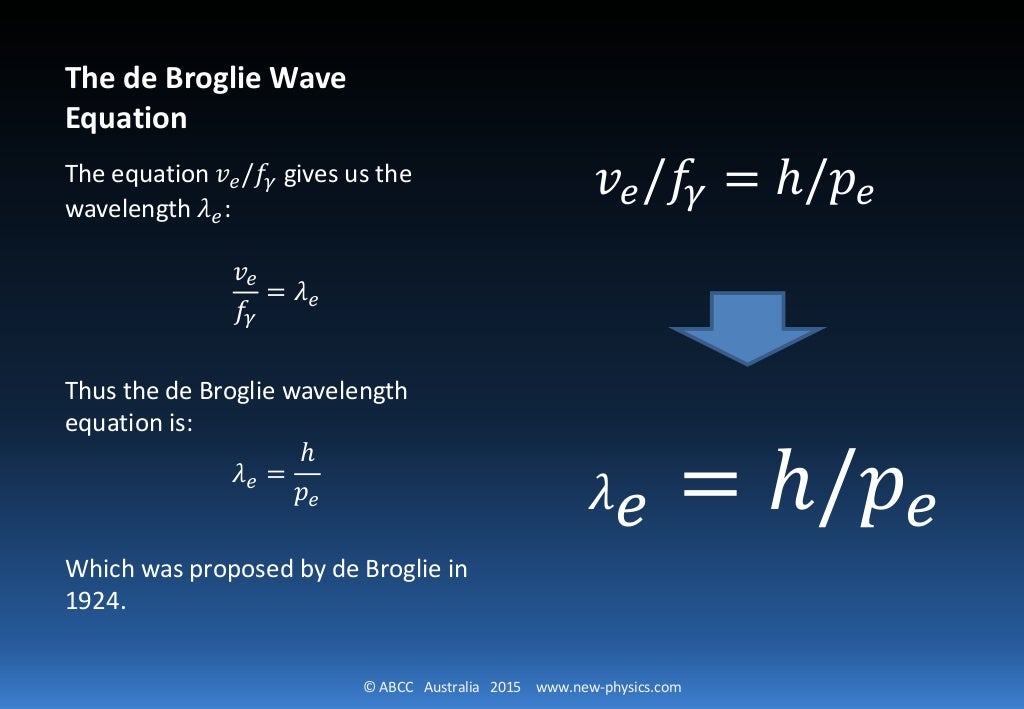
Now comes the second equation. It is one of two related equations called the de Broglie equations. You can read more about de Broglie's work here. He received the 1929 Nobel Prize in Physics for this work. (I will discuss the second de Broglie equation below the following example problems.) Equation Number Two: λ = h/p
De Broglie theory (Duality) Overall Science
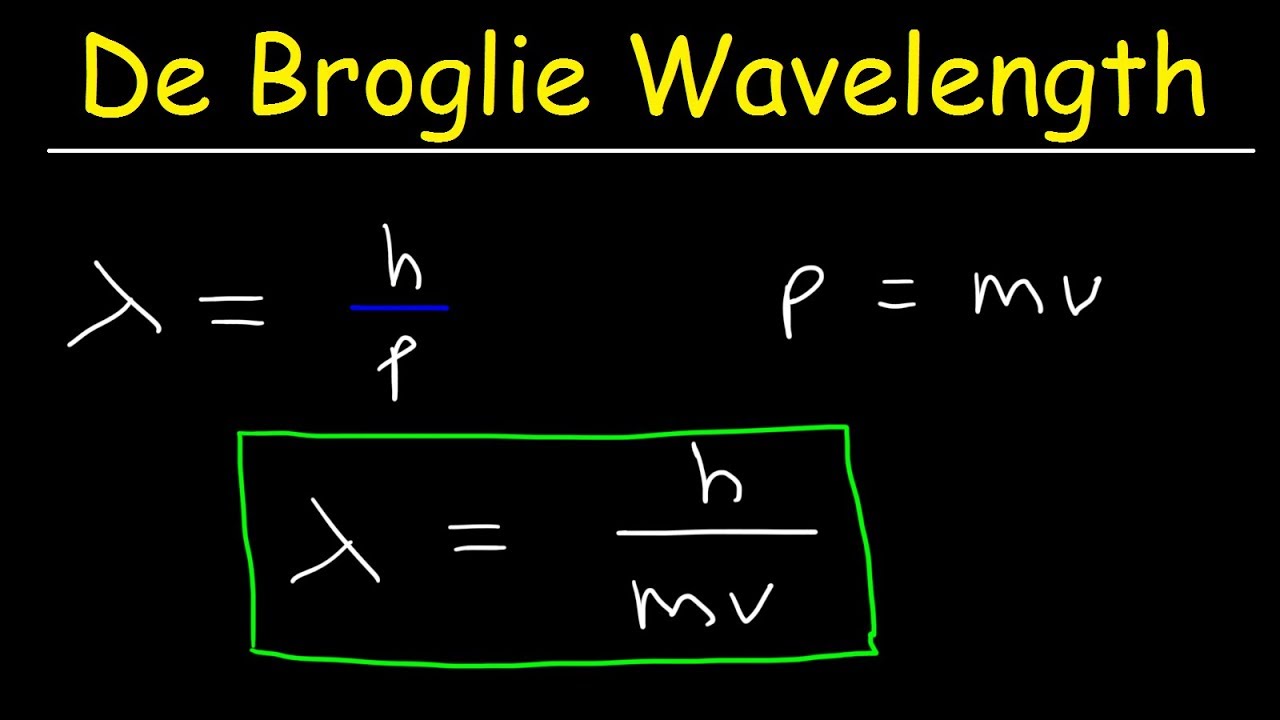
The de Broglie equation is an equation used to describe the wave properties of matter, specifically, the wave nature of the electron : λ = h/mv, where λ is wavelength, h is Planck's constant, m is the mass of a particle, moving at a velocity v. de Broglie suggested that particles can exhibit properties of waves.

PPT Chapter 27 PowerPoint Presentation, free download ID2929126
De Broglie Wavelength Formula is used to calculate the wavelength and momentum in any given problems based on this concept. Solved Examples Question 1: Find the wavelength of an electron moving with a speed of ms-1. Solution: Given: Velocity of the electron, v =2 ×106 ms-1 Mass of electron, m =9.1 ×10-31 Kg

de Broglie Equation — Overview & Calculations Expii
Is it a particle or a wave? This is the question that physicists of the 1920s were asking about light. In 1924, Louis de Broglie took this question to another level as he explored how electrons - which are matter, and were thought to be simply particles - can behave like waves. Questions Tips & Thanks Sort by: Top Voted Patrick 7 years ago
PM [D02] de Broglie deriving the Equation
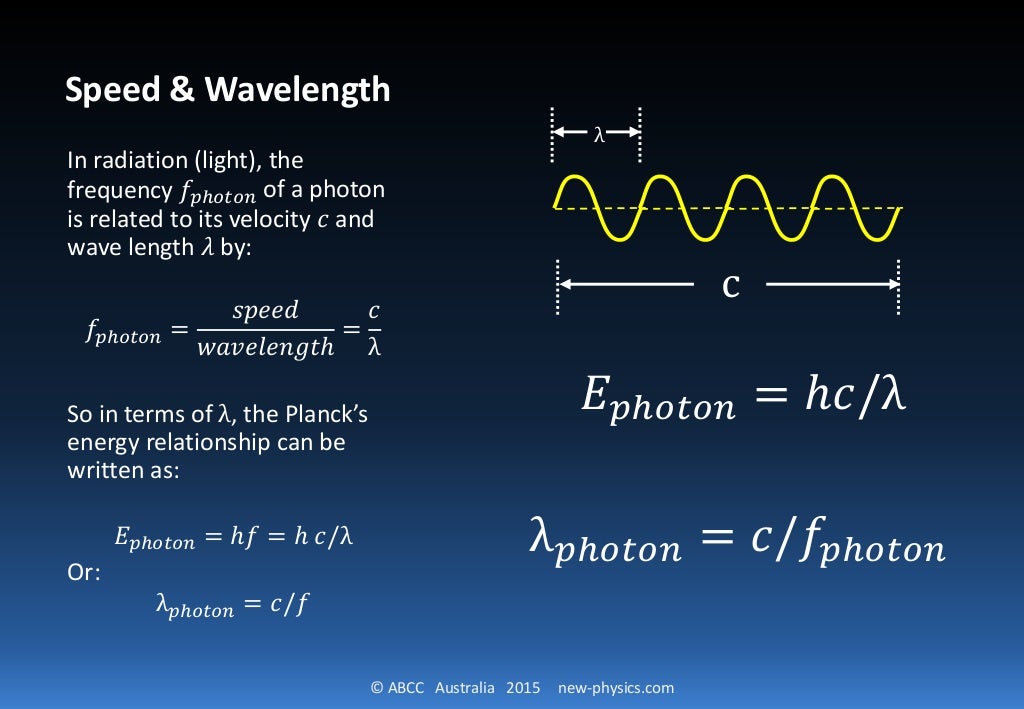
The De Broglie wavelength of a particle is derived by using the formulas for its energy. Consider a photon of mass m with energy as E, wavelength as λ and velocity equal to speed of light, c. The energy (E) of a photon is given as, E = hc/λ ⇢ (1) Also we know that, E = mc 2 ⇢ (2) Equating (1) and (2) we get, hc/λ = mc 2. h/λ = mc.
de Broglie equation Science, quantum theory ShowMe
Through the equation λ λ, de Broglie substituted v/λ v / λ for ν ν and arrived at the final expression that relates wavelength and particle with speed. mv2 = hv λ (5) (5) m v 2 = h v λ. Hence. λ = hv mv2 = h mv (6) (6) λ = h v m v 2 = h m v. A majority of Wave-Particle Duality problems are simple plug and chug via Equation 6 6 with.
What is the significance of de Broglie's equation? Quora
De Broglie Wavelength Formula is a formula that defines the nature of a wave to that of a particle. Many experiments show that light can behave both as a wave and as a particle. The particles of light are known as photons.

Structure Of Atom Concepts
De Broglie hypothesis Propagation of de Broglie waves in one dimension - real part of the complex amplitude is blue, imaginary part is green. The probability (shown as the color opacity) of finding the particle at a given point x is spread out like a waveform; there is no definite position of the particle.
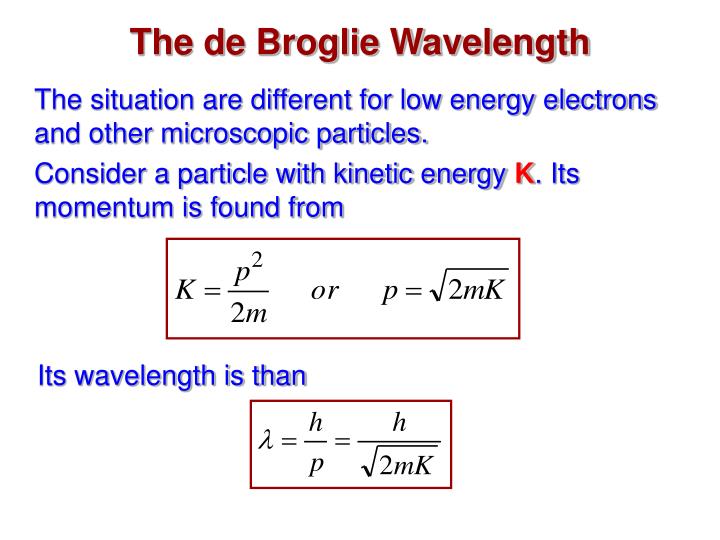
PPT Chapter 5 PowerPoint Presentation ID6630200
Compton's formula established that an electromagnetic wave can behave like a particle of light when interacting with matter. In 1924, Louis de Broglie proposed a new speculative hypothesis that electrons and other particles of matter can behave like waves. Today, this idea is known as de Broglie's hypothesis of matter waves.In 1926, De Broglie's hypothesis, together with Bohr's early.

Question Video Relating Momentum to the de Broglie Wavelength Nagwa
To determine the de Broglie wavelength of a particle given its mass and velocity, you need to: Multiply the velocity by mass. Their product is the particle's momentum. Divide Planck's constant by the momentum found in Step 1. The result you've got is exactly the de Broglie wavelength of your particle. Congrats!